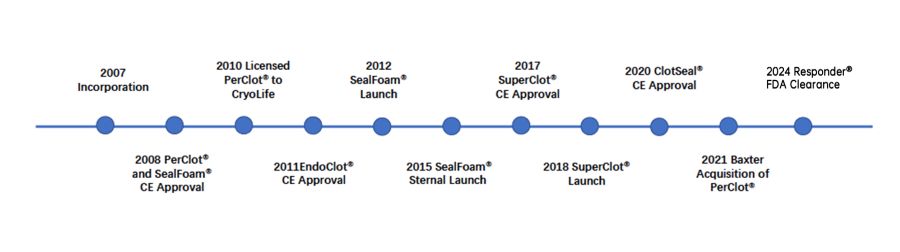
About Us
Starch Medical Inc. (SMI) is a San Jose, California based medical device company engaged in the design, manufacture and sale of innovative, absorbable hemostatic products. Our mission is to deliver advanced hemostatic technologies in a safe and effective manner to reduce bleeding-associated risks in surgeries and trauma.
SMI is committed to becoming an industry leader in surgical hemostats, providing surgeons a safe option for a diverse range of surgeries. The proprietary AMP® technology platform consists of biocompatible, ultra-hydrophilic polymer particles synthesized from purified plant starch. AMP® particles contain no human or animal components.
At SMI, we are dedicated to hemostatic excellence. This performance is reflected in our goal to provide customized systems to further improve the delivery of our break through AMP® technology. SMI is committed to supply the medical community with the most advanced and reliable hemostatic products.
Hemostasis Technology
AMP® is the Absorbable Modified Polymers technology platform that consists of biocompatible, ultra-hydrophilic and adhesive polymer particles synthesized from purified plant starch to control bleeding. AMP® materials contain no human or animal components. SuperClot®, PerClot®, SealFoam® and Responder® all incorporate the AMP® technology.
AMP® particles have a molecular structure that rapidly absorbs water from blood, causing a high concentration of platelets, red blood cells and coagulation proteins at the bleeding site, accelerating the physiologic clotting cascade. The interaction of AMP® with blood rapidly produces a gelled matrix that adheres to and seals the bleeding tissue. AMP® particles are readily dissolved by saline irrigation and are degraded rapidly by human enzymes, primarily amylase, within a few days.
SuperClot®, PerClot®, SealFoam® and Responder® patents are granted in the United States, Japan, India and China.
SuperClot®, SealFoam® are not approved for distribution in the United States. Responder® is approved in the United States.